Abstract
Steroid refractory acute graft versus host disease (SR-aGVHD) remains an area of unmet needs with more therapeutic options required. Here, we review novel treatment options for SR-aGVHD.
Methods:
A comprehensive data search was done across various data sets, including PubMed, Cochrane, and Embase, using MeSH terms and keywords to look for all the different drug types that have been studied for SR-aGVHD. A summary of the results is described here.
Results:
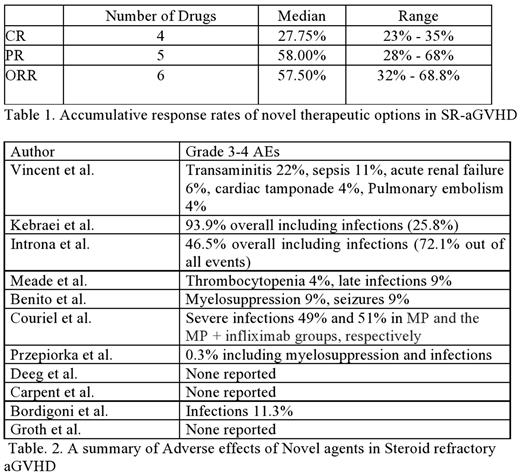
Denilekin diftitox (Ontak), a recombinant protein composed of IL-2 fused to diphtheria toxin, was studied in a phase 1 trial by Vincent et al. Thirty patients receiving 9 g/kg of Ontak at different intervals showed a Complete response (CR) of 38% and a partial response (PR) of 38%.
Remetemcel-L, ex vivo cultured adult human mesenchymal stem cells were studied in a multicenter phase III trial in 260 patients by Kebraei et al. compared to placebo. At a dose of 2 x 106 hMSC/kg, an overall response rate (ORR) of 58% vs. 54% in the placebo group was seen with CR of 35 and 30% respectively at day 28.
Third-party bone marrow-derived mesenchymal stromal cells (MSC) expanded in platelet lysate were studied by Introna et al. in a phase 1 trial. Forty patients received a median dose of 1.5 x 10(6)/kg per infusion and showed an ORR of 67.5% with a CR of 27.5%. OS at 1 and 2 years was 50% and 38.6%, respectively.
Pentostatin, an adenosine deaminase inhibitor, was studied by Meade et al. in a phase 1 trial in 23 patients. Pentostatin was administered at a dose of 1 to 4 mg/m2/day. Study showed a CR of 63%.
Sirolimus, an mTOR Inhibitor, was studied by Benito et al. in a pilot study in 21 patients. On an average dose of 5 mg/m2, sirolimus showed a CR of 23% and PR of 33%.
Couriel et al., in a phase III trial, 63 patients were randomized and received either methylprednisone (MP) 2mg/kg or infliximab plus MP. On days 7 and 28 response rate for infliximab + MP vs. MP was 52% vs. 78% and 62% vs. 58%, respectively.
Przepiorka et al. reported the results of an open-label phase II study in 20 patients with SR-aGVHD treated with BTI-322 (LO-CD2a), an anti-human CD2 SR-aGVHD IgG2b monoclonal antibody (MAB). ORR was achieved in 55% of the patients.
Deeg et al. resulted from a dose-escalation study in 59 patients with steroid-refractory aGVHD treated with ABX-CBL MAB. Reported ORR was 51%.
Carpent et al. reported the results of a phase II trial in which 44 patients were treated with visilizumab with ORR of 32% at day 42.
Bordigoni et al. treated 62 patients with daclizumab in a phase II trial with reportef ORR of 68.80% at 44 months follow up.
Groth et al. studied anti-CD3, anti CD7 antibodies separately conjugated to recombinant ricin A (CD3/CD7-1 T) in adults, 18 years and older with grade III to IV aGVHD following HSCT for myeloid and lymphoid malignancies. Twenty patients were treated with an ORR of 60% on day 28.
Adverse events are listed in the Table 2.
Conclusion
Different targeted drugs focused on the specific pathways show promising results. Monoclonal antibodies in particular showed good treatment outcomes with tolerable safety profile. Further studies are required to identify a valuable therapeutic option, SR-aGVHD.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal